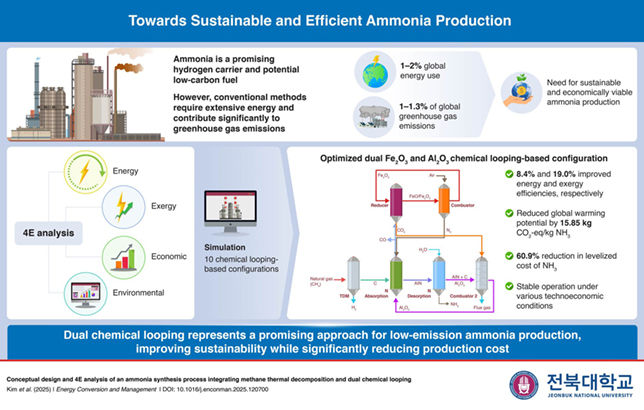
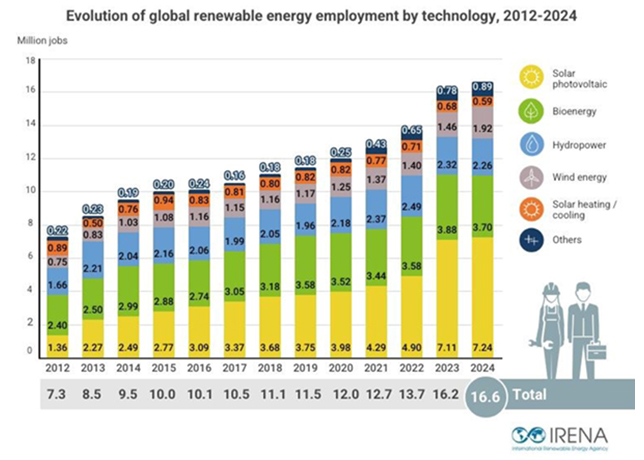
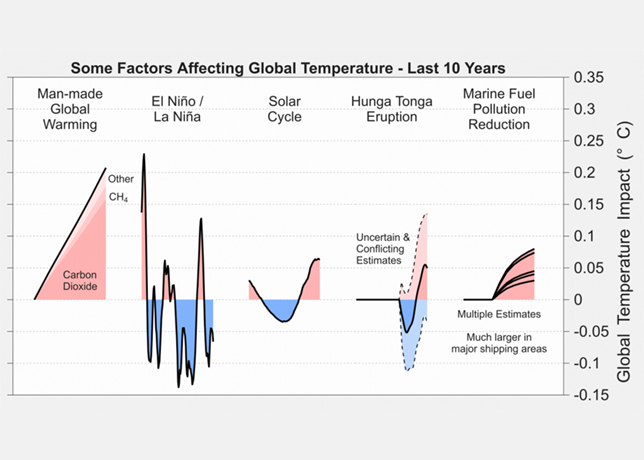
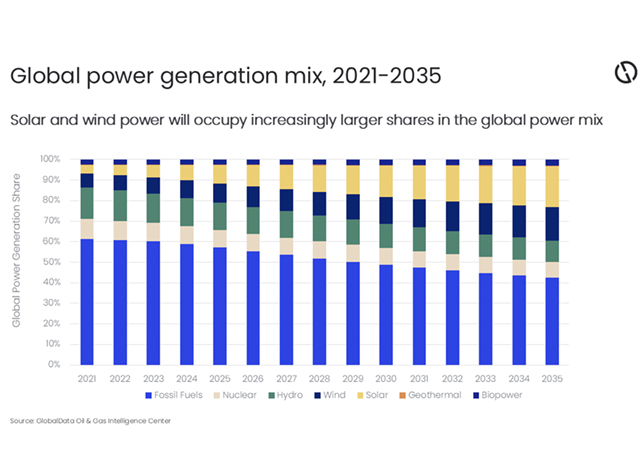
Recycling spent catalysts offer numerous economic and environmental benefits
Recycling spent catalysts offer numerous economic and environmental benefits
The economic and environmental benefits of recycling catalysts are significant. While recycling them can generate revenue, it also reduces the need for mining and processing new raw materials
Catalysts play an indispensable role in various industrial processes, enhancing reaction rates and efficiencies without being consumed in the process. However, over time, catalysts degrade and lose their effectiveness.
Recycling these spent catalysts instead of relegating them to landfills offers numerous economic and environmental benefits.
This practice not only mitigates environmental harm but also turns potential waste into valuable resources.
UNDERSTANDING CATALYSTS
Catalysts are substances that speed up chemical reactions without undergoing permanent changes themselves. They are crucial in industries ranging from petrochemicals to pharmaceuticals.
Catalysts come in two primary forms: homogeneous and heterogeneous. Homogeneous catalysts exist in the same phase (liquid or gas) as the reactants, while heterogeneous catalysts are in a different phase, typically solid catalysts in a liquid reaction mixture.
Each type has its advantages, with homogeneous catalysts offering high specificity and ease of separation, and heterogeneous catalysts providing straightforward separation and reuse.
ECONOMIC & ENVIRONMENTAL BENEFITS OF RECYCLING CATALYSTS
The economic incentives for recycling catalysts are significant. Disposing of spent catalysts in landfills incurs costs, while recycling them can generate revenue.
The metals commonly recycled from catalysts, such as molybdenum, tungsten, nickel, cobalt, copper, zinc, vanadium, platinum, and palladium, have substantial market value. For instance, as of May 2023, reclaimed molybdenum is valued at $21 per pound, and nickel fetches nearly $10 per pound.
By recycling these metals, industries can reduce the cost of producing new catalysts, as recovered metals serve as raw materials.
This cost-saving aspect is particularly vital as it lowers the expenses for future catalyst loads.
Moreover, businesses that adopt recycling practices can enhance their market reputation, attracting environmentally conscious customers and gaining a competitive edge.
Recycling catalysts offers profound environmental benefits. It reduces the need for mining and processing new raw materials, thus conserving natural resources and decreasing environmental degradation.
The energy savings from recycling are substantial, leading to lower greenhouse gas emissions and mitigating climate change impacts.
Additionally, recycling keeps hazardous materials out of landfills, preventing soil and water contamination.
The conservation of precious metals through recycling also contributes to sustainable resource management. By reusing these materials, industries can help ensure their availability for future generations, supporting long-term economic and environmental sustainability.
HOMOGENEOUS VS HETEROGENEOUS CATALYSTS
The distinction between homogeneous and heterogeneous catalysts lies in their phase relative to the reactants.
Homogeneous catalysts, often used in liquid-phase reactions, offer advantages in terms of uniform reaction conditions and ease of molecular interaction.
This can lead to high reaction rates and selectivity. In contrast, heterogeneous catalysts, usually solids, are preferred for their ease of separation from reaction mixtures and robustness in various industrial processes.
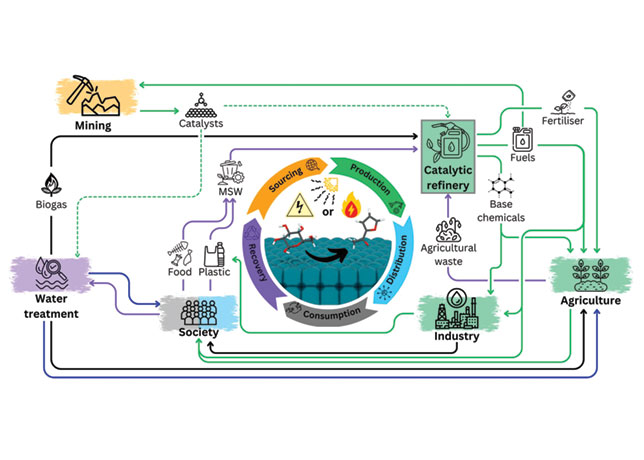
A notable advancement in the field of catalyst recycling comes from researchers at The Max Planck Society in Germany. Their work focuses on establishing basic principles and guidelines for experiments and evaluations of catalyst recycling, particularly for organometallic complexes.
These guidelines are not limited to organometallic catalysts but are also applicable to other homogeneously dispersed catalytic species, including nanoparticles and enzymes.
The researchers emphasise the importance of reliable and generalised methods for recycling experiments at the laboratory scale.
These methods are crucial for collecting valuable data that allow for the conclusive comparison of different recycling approaches.
Their study categorises industrially applied techniques for recycling organometallic catalysts and evaluates performance indicators of recycling methods at the research stage.
The principles outlined by researchers include integrating recycling into industrial processes to achieve quasi-stationary operation, optimising catalyst design for easier separations, and ensuring efficient utilisation of catalytic components.
They highlight the need for balancing productivity and selectivity with recyclability.
While highly selective ligands may offer superior performance, their challenging recyclability can diminish overall utility.
Conversely, simpler catalysts that are easier to recycle can enhance process efficiency.
Their perspective review encompasses industrial recycling strategies and categorises them based on their applicability and effectiveness.
For instance, the recycling of highly active metallocene catalysts used in large-scale processes, such as olefin polymerisation, might be less imperative due to their inherent efficiency and cost-effectiveness.
CONCLUSION
Catalyst recycling represents a critical intersection of economic viability and environmental stewardship.
By recovering valuable metals from spent catalysts, industries can reduce costs, conserve natural resources, and minimise environmental impacts.
The work by The Max Planck Society underscores the importance of standardised methods for recycling experiments, paving the way for more effective and sustainable industrial practices.
As we move towards a more sustainable future, the recycling of catalysts will play an increasingly vital role.
It offers a pragmatic solution to resource conservation and environmental protection, ensuring that the benefits of catalytic processes are maximised while minimising their ecological footprint.