New catalytic systems and techniques are needed to reduce GHG gas emissions
New catalytic systems and techniques are needed to reduce GHG gas emissions
The chemical sciences and engineering communities are actively pursuing new strategies to reduce the negative impacts of industrial processes. Many of these necessary chemical reactions involve catalytic pathways
A major effort in the manufacturing and chemical industry is developing new catalytic systems and techniques to improve atom efficiency, energy efficiency, and the reduction of greenhouse gas (GHG) emissions.
These strategies centre on decreasing the use of thermal energy sources and increasing the development of alternative methods for energy delivery.
Researchers at ‘Vistas in Catalysis’, a session within a workshop, titled ‘Innovations in Catalysis to Address Modern Challenges’ sought to explore innovative solutions from a range of perspectives with the potential to make significant improvements in chemical production. The workshop was conducted by the National Academies of Sciences, Engineering, and Medicine.
• In their presentation on ‘Electrocatalytic Ammonia Oxidation by a Low Coordinate Copper Complex’ Estak Ahmed and his colleagues from the Michigan State University stressed a growing need for catalysts that electrocatalytically oxidise ammonia for fuel cells or on-demand hydrogen production that produce only nitrogen (N2) as a byproduct.
"While hydrogen has attracted much attention, practical limitations connected to distribution challenges call for alternative approaches. Owing to its high energy density and established global production and distribution networks, ammonia (NH3) is an especially appealing energy carrier," they said in their abstract.
They described the electrocatalytic oxidation of ammonia using molecular complexes based on earth-abundant copper.
• Considering that the development of new catalytic systems is a time-intensive and expensive challenge, Daniela Blanco from Sunthetics said data-driven machine-learning (ML) offers the possibility to dramatically reduce the resources needed for reaction optimisation through supervised-learning models that predict reaction performance, catalytic behavior, and optimal reaction conditions.
However, most ML algorithms require large datasets (hundreds or thousands of points) to provide accurate predictions, while experimental data collection is expensive in human and material resources.
But the challenge of building large datasets can be averted with Bayesian Optimization frameworks that guide experimental campaigns through informed and smart decisions, Blanco noted in the abstract of her paper, ‘Leveraging Small Data for Faster and Better Catalysis’.
"We introduce a modified Bayesian Optimization approach that accelerates the design and implementation of intelligent experimental campaigns for faster and better catalytic transformations. Working at the intersection of ML and chemistry, we created SuntheticsML, an online, user-friendly, ML platform that leverages very small data (starting with only 5 data points) to enable the development of new catalytic processes, materials, and formulations up to 15 times faster without the need of coding or ML skills," she said.
SuntheticsML accelerates lengthy and complex traditional optimisation campaigns, harnessing the power of ML and small data to fast-track innovation, sustainability, and digitalisation in the chemical industry, further reducing development waste, emissions, and resource consumption by up to 95 per cent.
• Long Qi from the Ames National Laboratory reported the nitrogen-assembly carbons (NACs) as the first metal-free catalyst achieving room temperature dihydrogen dissociation and subsequent application in transformation of bio-derived oxygenates.
Transition metal-containing catalysts are employed in hydrotreatment although accompanied with poor selectivity.
In his presentation on ‘New Catalytic Mechanisms for Strong Bond Activation without Transition Metals’, Qi further showed that NAC catalysts are versatile for activating heterocycles as liquid organic hydrogen carriers (LOHCs) to release hydrogen.
The discovery of nitrogen assembly as active sites can open broad opportunities for rational design of new metal-free catalysts for challenging chemical reactions for a carbon-neutral future.
• In their paper, ‘Linear paired electrochemical valorisation of glycerol enabled by the electro-Fenton process using a stable NiSe2 cathode, Dr Hongyuan Sheng and his team from University of California, Los Angeles, reasoned that electrochemical valorisation of surplus biomass-derived feedstocks, such as glycerol, into high-value chemicals offers a sustainable route for utilisation of biomass resources and decarbonization of chemical manufacturing. However, glycerol is typically valorised solely via anodic oxidation, with lower-value products such as hydrogen gas generated at the cathode.
The researchers established the efficient cathodic valorisation of glycerol to the desirable C3 oxidation products via the electro-fenton process at a stable NiSe2 cathode, built upon the theoretical understanding and experimental demonstration of the high selectivity and stability of NiSe2 toward acidic H2O2 electrosynthesis.
• In their paper, ‘Tandem Catalysis for Rapid Decarbonization of Chemical Manufacturing’, Samay Garg and his team from Columbia University present an overview of tandem electrocatalytic-thermocatalytic processes for sustainable synthesis of high-volume commodity chemicals, including C3 oxygenates and aromatics.
Sustainable processes for chemical synthesis are vital as attempts are made to limit global warming and avoid the worst effects of climate change.
The electrochemical CO2 reduction reaction (CO2RR) has been widely studied in recent years as a promising strategy for reducing atmospheric CO2 concentration via both decarbonisation and CO2 utilisation, but real-world implementation of electrochemical CO2RR is limited by low selectivities for valuable multicarbon products.
Many well-established thermochemical processes, however, use simple CO2RR products (for example, CO and H2) as reactants.
By coupling an initial electrocatalytic reaction with a downstream thermocatalytic reaction, it is possible to achieve high selectivities for the desired products at commercially relevant production rates.
Optimising electrochemical CO2RR in the context of this tandem system, rather pursuing high single-single pass selectivities, will accelerate the deployment of electrochemical CO2RR.
Furthermore, this tandem approach can be applied to produce molecules that are more complex than can be produced via direct electrochemical CO2RR.
• Extraordinarily efforts have been placed on developing novel electrocatalysts for transforming energy relevant small molecules. Catalysts particles are stabilised by surfactants and supported on carbon materials.
The roles of these molecular additives and heterogeneous supports are often considered negligible or worse yet, not considered at all.
In their paper, titled ‘Novel Molecular Strategies to Modulate the Electrode-Electrolyte Interface in Heterogeneous Electrocatalysis’ Sarah Thoi and her research team from Johns Hopkins University have demonstrated that cationic surfactants play a significant role in modulating the electrode-electrolyte interface during carbon dioxide reduction.
"Moreover, we will present new strategies to probe the electrode-electrolyte interfaces in electrocatalysis using advanced electrochemical techniques such as in-situ vibrational spectroscopy and electrochemical impedance spectroscopy. Our studies highlight molecular strategies to tune complex heterogeneous interfaces and opens up new avenues for directing catalytic performance," she said.
• The research of Emily Ann Sprague-Klein from Brown University on ‘Light-Induced Chemical Structure Dynamics in Transition Metal Oxides for Photocatalysis’ focuses on developing a fundamental understanding of visible light-activated excited state chemistry that is of great importance in elucidating the mechanisms, structures, and design features for catalysis.
"Solar energy harvesting is a nuanced scientific field that involves precise control across energy, time, and nanometer length scales. The understanding of how to harness sunlight to promote chemical reactions is vital for the advancement of renewable energy applications and a sustainable future," she wrote in the abstract.
"Limited knowledge of the precise chemical mechanisms underlying solar energy harvesting restricts widespread technological use and the development of photovoltaic devices that utilise light-harvesting biodegradable materials."
Sprague-Klein examines excited-state chemical structure dynamics in nanomaterials that use light-matter interactions to drive interfacial or intramolecular charge transfer. Steady-state and timeresolved optical/X-ray spectroscopy allow for the observation of these dynamics across an expansive timescale—from the seconds timescale of oxygen-evolving reactions to the ultrafast timescales of electronic and vibrational motion.
• Wesley Chang from the California Institute of Technology said his work on ‘Electrochemical Nitrogen Reduction’ will further enable commercially viable lithium-mediated nitrogen reduction electrocatalysis.
Lithium metal is unique in its ability to reduce nitrogen, and the process involves the formation of a complicated interphase that allows for both nitrogen and proton transport to selectively form ammonia.
However, this system is limited by the chemical reactivity of lithium and electrolyte oxidation on the anode.
In his paper, Chang described his efforts in Dr Karthish Manthiram’s lab at Caltech to mitigate electrolyte oxidation through the use of a lithium-ion conductive solid electrolyte; scale up the system from 1 sq cm to a commercially relevant 10 sq cm working electrode area demonstrating consistent ammonia Faradaic efficiency; further understand the lithium interphase using materials characterization techniques.
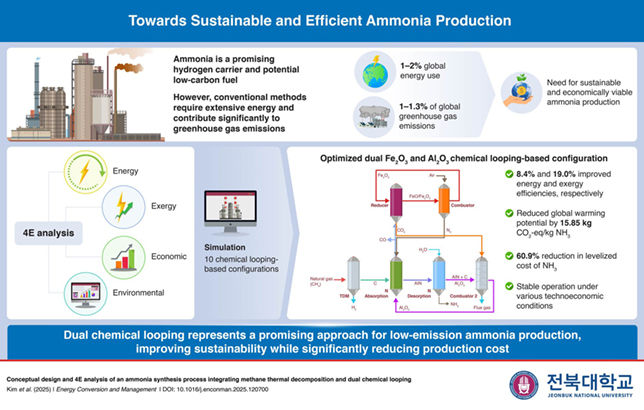
The production of ammonia as an industrial chemical contributes up to 10 per cent of global carbon emissions and requires around 1 per cent of global energy consumption.
Recently, the use of electricity to generate ammonia at room temperature and ambient pressure has been demonstrated.