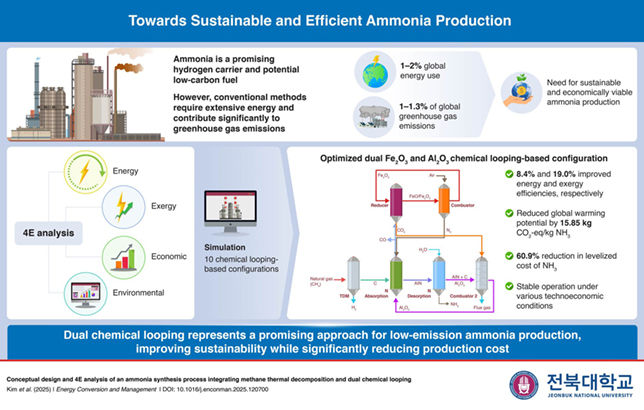
 The proposed dual-chemical looping strategy
The proposed dual-chemical looping strategy
As the world moves toward cleaner energy systems, this process could support future clean-fuel applications and broader carbon-neutral energy transition strategies, says Dr Sunghyun Cho
Ammonia is an essential chemical used across many industries worldwide. Beyond its traditional role as a fertiliser, it is also a promising liquid hydrogen carrier and low-carbon fuel that could help reduce reliance on fossil fuels.
With a high hydrogen content of 17.8 percent by weight and volumetric energy density of 12.7 MJ/L, ammonia supports long-distance transport and storage, while its combustion produces no direct CO2 emissions.
Global demand for ammonia is projected to rise by about 20 per cent by 2030, potentially tripling or quadrupling to 560-665 million metric tons per annum (mtpa) by 2050 under a 1.5 deg C climate scenario.
However, conventional ammonia production based on the Haber-Bosch (HB) process requires considerable energy and contributes significantly to greenhouse gas emissions, accounting for roughly 1-1.3 per cent of global emissions annually.
Relying on hydrogen from steam methane reforming (SMR) or coal gasification, it emits 2.5-2.9 kg CO2-eq/kg NH2 for SMR and up to 5.2 kg CO2-eq/kg NH3 for coal, consuming 1-2 per cent of global energy under harsh conditions of 400-500 deg C and 150-250 bar.
Given its growing importance, there is an urgent need to reduce the environmental burden of ammonia production.
Recently, chemical looping ammonia synthesis (CLAS) has emerged as a viable alternative method for ammonia production.
This approach uses redox cycles with metal oxides to separate and react gases, enabling lower temperatures and intrinsic CO2 capture.
Studies on Fe-based carriers show high reactivity and stability, while Al-based systems fix nitrogen effectively at high temperatures.
Specifically, aluminium-oxide (Al2O3)-based chemical looping has shown promise for enabling ammonia synthesis under more energy-efficient conditions.
Research highlights Cr- or AD-modified alumina for enhanced N-fixation above 1,300 deg C with good cyclic durability.
Building on this concept, a research team led by Dr Sunghyun Cho from the School of Chemical Engineering, School of Semiconductor and Chemical Engineering at Jeonbuk National University in South Korea, has developed a new dual-chemical looping process combining Al2O3 and iron oxide (Fe2O3).
In their paper titled, ‘Conceptual design and 4E analysis of an ammonia synthesis process integrating methane thermal decomposition and dual chemical looping’, and published in the Energy Conversion and Management Journal, his team through an integrated 4E analysis showed improved efficiency and robustness while reducing production costs.
Their dual chemical looping strategy for ammonia synthesis avoids several energy-intensive steps used in traditional processes.
By integrating TDM, which decomposes CH4 into solid carbon and H2 at over 1,000 deg C (or lower with catalysts), the process uses carbon as a reductant in A-CL, avoiding extra CO2.
F-CL, in two- or three-reactor setups, reduces Fe2O3with CO/H2 to FeO/Fe2O4, oxidises with steam for H2, and regenerates with air for N2.
"Our approach combines methane thermal decomposition with Al2O3- and Fe2O3-based chemical looping cycles," explains Dr Cho.
"This method enables ammonia synthesis without the energy-intensive steps, significantly improving both sustainability and efficiency."
The Al2O3-based chemical loop (A-CL) has two stages: Nitrogen fixation and hydrolysis.
During nitrogen fixation, Al2O3 combines with nitrogen and solid carbon to generate aluminium nitride (AlN).
This occurs at 1,300–2,000 deg C, with CO as the main gaseous product, thermodynamically favorable above 1,500 deg C.
This is followed by hydrolysis, where AlN interacts with steam to produce ammonia. Modeled with LHHW kinetics, it regenerates Al2O3 efficiently.
In the proposed dual-looping process, A-CL is complemented by thermal decomposition of methane (TDM) and an Fe2O3-based chemical loop (F-CL).
TDM supplies solid carbon for A-CL, while F-CL provides nitrogen, eliminating the need for additional air separation units.
F-CL reactions include stepwise reduction (Fe2O3 to Fe3O4/FeO) and regeneration, producing high-purity N2 (from depleted air) and H2.
Additionally, carbon monoxide generation within A-CL provides reusable feedstock for F-CL systems.
Together, these interactions create a cross-linked circulation of key feedstock materials.
Simulations validated TDM kinetics against experimental data, showing good agreement in CH4 conversion.
To test practical feasibility, the researchers conducted a comprehensive energy, exergy, economic, and environmental (4E) analysis of 10 different process configurations, including the proposed dual-looping system and its modified variations, the conventional HB process, and both Al2O3-based and Fe2O3-based single-chemical looping systems.
Simulation results showed that the proposed configuration outperformed conventional production methods in both energy and exergy efficiencies by 8.4 per cent and 19 per cent, respectively.
It reduced global warming potential by up to 15.85 kg of CO2-equivalent per kg of ammonia produced.
It also demonstrated the lowest production costs among all evaluated cases. Sensitivity analysis further confirmed its robustness under varying technoeconomic conditions.
Sensitivity analysis further confirmed its robustness under varying technoeconomic conditions.
The levelised cost dropped to 336.97 USD/t NH3, a 60.9 per cent reduction, due to byproduct valorisation and integrated flows.
Notably, integrating heat exchangers significantly improved energy and exergy efficiencies of all configurations.
"Our dual-looping technology can be applied across industries that require large-scale ammonia production while reducing carbon emissions and maintaining economic feasibility," concludes Dr Cho.
"As the world moves toward cleaner energy systems, this process could support future clean-fuel applications and broader carbon-neutral energy-transition strategies."